In the waning days of World War II, a little-known Austrian inventor did something unthinkable — he made water defy gravity.

His name? Viktor Schauberger, a self-taught forest caretaker and natural scientist who believed nature held secrets far beyond conventional physics. But what he discovered in 1945 didn’t just stir scientific curiosity — it triggered a global intelligence war.
🔬 Anti-Gravity Water?
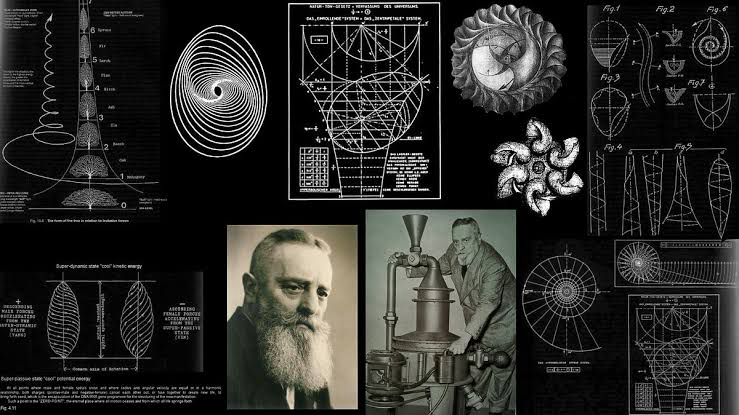
Schauberger’s invention was based on vortex dynamics — the swirling motion of water he observed in mountain streams. He claimed that, under certain conditions, water could generate levitational forces. In one of his experiments, a fluid vortex lifted objects upward — seemingly breaking the laws of gravity.
⚙️ His Device: The Repulsine
He built a machine called the Repulsine — a bell-shaped craft that used spinning vortex chambers. According to reports, it produced lift and propulsion without fuel. Many believe it may have been a prototype for anti-gravity flight.
☠️ Enter: Hitler’s SS

Once word of his discovery reached high command, Hitler’s SS abducted Schauberger and forced him into secret weapon projects. He worked under brutal conditions in a hidden Austrian lab — allegedly contributing to Nazi UFO experiments during the final days of the Third Reich.
🇺🇸 Post-War Secrecy

After Germany’s fall, Operation Paperclip brought top Nazi scientists to the U.S. Schauberger was brought in too — under duress. He was interrogated, and his inventions were immediately classified. The U.S. military sealed his documents, many of which remain restricted even today.
🕵️♂️ Spies and the Soviet Hunt

The Soviets, hearing whispers of an “anti-gravity weapon,” sent agents to locate his work. Some reports suggest KGB-backed scientists tried to reverse engineer Schauberger’s blueprints, believing they held the key to a new arms race.
🧪 His Darkest Discovery
But Schauberger’s real terror wasn’t about levitation. Before he died in 1958, he hinted that he had stumbled upon a force so destructive it could alter Earth’s balance — a form of natural energy capable of planetary devastation if misused.
🗝️ What Was Buried… May Be Unearthed

Declassified files slowly emerging hint at technologies decades ahead of their time, tied to vortex physics and clean energy. Was Schauberger a madman? Or a genius silenced by governments?
One thing is clear: His story is not finished. And perhaps… neither is his invention.